Abstract
Purpose
This study compared 68Gallium-prostate-specific-membrane-antigen based Positron-emission-tomography (68Ga-PSMA-PET) and 99metastabletechnetium-3,3-diphospho-1,2-propanedicarbonacid (99mTc-DPD-SPECT) in performing skeletal staging in prostate cancer (PC) patients and evaluated the additional value of the information from low-dose-computed tomography (CT).
Materials and Methods
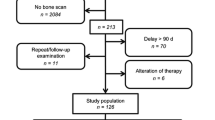
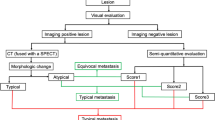
In this retrospective study, 54 patients who received 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT within 80 days were extracted from our database. Osseous lesions were classified as benign, malignant or equivocal. Lesion, region and patient based analysis was performed with and without CT fusion. The reference standard was generated by defining a best valuable comparator (BVC) containing information from all available data.
Results
In the patient based analysis, accuracies measured as “area-under-the-curve” (AUC) for 68Ga-PSMA-PET, 99mTc-SPECT, 68Ga-PSMA-PET/CT and 99mTc-SPECT/CT were 0.97-0.96, 0.86-0.83, 1.00 and 0.83, respectively (p<0.05) (ranges = optimistic vs. pessimistic view). Region based analysis resulted in the following sensitivities and specificities: 91.8-97.7%, 100-99.5% (PET); 61.2-70.6%, 99.8-98.3% (SPECT); 97.7%, 100% (PET/CT), 69.4% and 98.3% (SPECT/CT) (p<0.05). The amount of correct classifications of equivocal lesions by CT was significantly higher in PET (100%) compared to SPECT (52.4%) (p<0.05).
Conclusion
68Ga-PSMA-PET outperforms 99mTc-DPD-SPECT in detecting bone metastases in PC patients. Additional information from low-dose-CT resulted in a significant reduction in equivocal lesions in both modalities, however 68Ga-PSMA-PET benefited most.
Key Points
• Ga-PSMA-PET outperforms 99m Tc-DPD-SPECT in skeletal staging in prostate cancer patients
• Proportion of equivocal decisions was significantly reduced by CT-fusion in both modalities
• Ga-PSMA-PET benefits more from CT information, compared to 99m Tc-DPD-SPECT




Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- CT:
-
computed tomography
- BVC:
-
best valuable comparator
- BS:
-
Bone scan
- MRI:
-
Magnetic resonance imaging
- PC:
-
Prostate cancer
- Tc-DPD:
-
99-technetium-3,3-Diphospho-1,2-propanedicarbonacid
- PSMA:
-
Prostate specific membrane antigen
- Ga-PSMA-HBED-CC:
-
prostate specific membrane antigen (PSMA) ligand (Glu-NH-CO-NH-Lys) radio-labelled with 68Gallium-N,N-bis[2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N diacetic acid
- Ga-PSMA-PET:
-
68Gallium prostate-specific-membrane-antigen based positron-emission-tomography
- DPD:
-
Diphospho-1,2-propanedicarbonacid
- PACS:
-
Picture archiving and communication system
- CI:
-
confidence interval
- PSA:
-
prostate specific antigen
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristics
- HU:
-
Hounsfield unit
- 2D:
-
two dimensional
- 3D:
-
three dimensional
- MIP:
-
maximum intensity projection
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Bubendorf L, Schopfer A, Wagner U et al (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31:578–583
Carlin BI, Andriole GL (2000) The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer 88:2989–2994
Rigaud J, Tiguert R, Le Normand L et al (2002) Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol 168:1423–1426
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Mohler JL, Kantoff PW, Armstrong AJ et al (2014) Prostate cancer, version 2.2014. J Natl Compr Canc Netw 12:686–718
Thompson I, Thrasher JB, Aus G et al (2007) Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 177:2106–2131
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I (2006) The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 47:287–297
Schirrmeister H, Glatting G, Hetzel J et al (2001) Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med 42:1800–1804
Apostolova I, Golcuk E, Bohuslavizki KH, Buchert R, Brenner W (2009) Impact of additional SPECT in bone scanning in tumor patients with suspected metastatic bone disease. Ann Nucl Med 23:869–875
Eder M, Neels O, Muller M et al (2014) Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals (Basel) 7:779–796
Afshar-Oromieh A, Malcher A, Eder M et al (2013) PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 40:486–495
Eder M, Schafer M, Bauder-Wust U et al (2012) 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 23:688–697
Eiber M, Maurer T, Souvatzoglou M et al (2015) Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med 56:668–674
Maurer T, Gschwend JE, Rauscher I et al (2016) Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 195:1436–1443
Afshar-Oromieh A, Avtzi E, Giesel FL et al (2015) The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42:197–209
Rowe SP, Gorin MA, Allaf ME et al (2016) PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis 19:223–230
Pyka T, Okamoto S, Dahlbender M et al (2016) Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 43:2114–2121
Dietlein M, Kobe C, Kuhnert G et al (2015) Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol Imaging Biol 17:575–584
Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS (2007) Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 48:471–480
Lecouvet FE, El Mouedden J, Collette L et al (2012) Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 62:68–75
Woo S, Kim SY, Kim SH, Cho JY (2016) JOURNAL CLUB: Identification of Bone Metastasis With Routine Prostate MRI: A Study of Patients With Newly Diagnosed Prostate Cancer. AJR Am J Roentgenol 206:1156–1163
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Richardson JT (2011) The analysis of 2 x 2 contingency tables--yet again. Stat Med 30:890, author reply 891-892
Campbell I (2007) Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med 26:3661–3675
Jambor I, Kuisma A, Ramadan S et al (2016) Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol 55:59–67
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM (2012) [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging 39:1085–1086
Francis MDHP, Tofe AJ (1981) Controversial mechanism of technetium-99m deposition on bone. J Nucl Med 22:72
Jones AG, Francis MD, Davis MA (1976) Bone scanning: radionuclidic reaction mechanisms. Semin Nucl Med 6:3–18
Eder M, Eisenhut M, Babich J, Haberkorn U (2013) PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging 40:819–823
Blazak JK, Thomas P (2016) Paget Disease: A Potential Pitfall in PSMA PET for Prostate Cancer. Clin Nucl Med 41:699–700
Sasikumar A, Joy A, Pillai MR, Raman V, Vasudevan A, Madhavan J (2017) A rare case of rectal carcinoma and prostate carcinoma with coexistent Paget's disease mimicking bone metastases in both 18F-FDG and 68Ga PSMA PET/CT. Eur J Nucl Med Mol Imaging 44:173
Lecouvet FE, Geukens D, Stainier A et al (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25:3281–3287
Pasoglou V, Larbi A, Collette L et al (2014) One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified "all-in-one" imaging approach? Prostate 74:469–477
Acknowledgements
The author MM is grateful for the financial support from the Deutsche Forschungsgemeinschaft (DFG, 5943/31/41/91).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jan-Carlo Janssen.
Conflict of interest
Dr. Hamm declares a relationship with the following companies, including: GE, Guerbet, Siemens, Samsung und Toshiba.
In addition to the author Hamm, no other author has a conflict of interest.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
Daniel Schulze kindly provided statistical advice for this manuscript.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
In this retrospective study, written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Janssen, JC., Meißner, S., Woythal, N. et al. Comparison of hybrid 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT . Eur Radiol 28, 610–619 (2018). https://doi.org/10.1007/s00330-017-4994-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4994-6