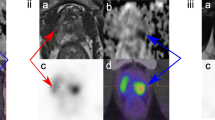
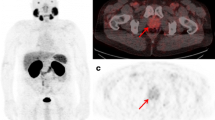
Abstract
Objectives
To quantitatively characterize clinically significant intra-prostatic cancer (IPC) by prostate-specific membrane antigen (PSMA) expression and cell density on PSMA-11 positron emission tomography/magnetic resonance (PET/MR).
Methods
Retrospective study approved by the institutional review board with informed written consent obtained. Patients with a solitary, biopsy-proven prostate cancer, Gleason score (GS) ≥7, presenting for initial evaluation by PET/computerised tomography (PET/CT), underwent early prostate PET/MR immediately after PSMA-11 tracer injection. PET/MR [MRI-based attenuation correction (MRAC)] and PET/CT [CT-based AC (CTAC)] maximal standardised uptake value (SUVmax) and minimal and mean apparent diffusion coefficient (ADCmin, ADCmean; respectively) in normal prostatic tissue (NPT) were compared to IPC area. The relationship between SUVmax, ADCmin and ADCmean measurements was obtained.
Results
Twenty-two patients (mean age 69.5±5.0 years) were included in the analysis. Forty-four prostate areas were evaluated (22 IPC and 22 NPT). Median MRAC SUVmax of NPT was significantly lower than median MRAC SUVmax of IPC (p < 0.0001). Median ADCmin and ADCmean of NPT was significantly higher than median ADCmin and ADCmean of IPC (p < 0.0001). A very good correlation was found between MRAC SUVmax with CTAC SUVmax (rho = –0.843, p < 0.0001). A good inverse relationship was found between MRAC SUVmax and CTAC SUVmax with ADCmin (rho = –0.717, p < 0.0001 and –0.740, p < 0.0001; respectively; Z = 0.22, p = 0.82, NS) and with MRAC SUVmax and ADCmean (rho = –0.737, p < 0.0001).
Conclusions
PET/MR SUVmax, ADCmin and ADCmean are distinct biomarkers able to differentiate between IPC and NPT in naïve prostate cancer patients with GS ≥ 7.
Key Points
• PSMA PET/MR metrics differentiate between normal and tumoural prostatic tissue.
• A multi-parametric approach combining molecular and anatomical information might direct prostate biopsy.
• PSMA PET/MR metrics are warranted for radiomics analysis.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- FWHM:
-
Full width at half maximum
- GS:
-
Gleason score
- IPC:
-
Intra-prostatic cancer
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- NPT:
-
Normal prostatic tissue
- OSEM:
-
Ordered subset expectation maximisation
- PET/MR:
-
Positron emission tomography/magnetic resonance
- PiRADS:
-
Prostate Imaging Reporting and Data System
- PSMA:
-
Prostate-specific membrane antigen
- ROI:
-
Region of interest
- SUV:
-
standardised uptake value
References
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol Biomarkers Prev 25(1):16–27
Carlaw KR, Woo HH (2017) Evaluation of the changing landscape of prostate cancer diagnosis and management from 2005 to 2016. Prostate Int 5(4):130–134
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol 69(1):41–49
Kasel-Seibert M, Lehmann T, Aschenbach R et al (2016) Assessment of PI-RADS v2 for the Detection of Prostate Cancer. Eur J Radiol 85(4):726–731
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: Images Are More than Pictures, They Are Data. Radiology 278(2):563–577
Wang J, Wu C-J, Bao M-L, Zhang J, Wang X-N, Zhang Y-D (2017) Machine learning-based analysis of MR radiomics can help to improve the diagnostic performance of PI-RADS v2 in clinically relevant prostate cancer. Eur Radiol 27(10):4082–4090
Stoyanova R, Takhar M, Tschudi Y et al (2016) Prostate cancer radiomics and the promise of radiogenomics. Transl Cancer Res 5(4):432–447
Fedorov A, Vangel MG, Tempany CM, Fennessy FM (2017) Multiparametric Magnetic Resonance Imaging of the Prostate: Repeatability of Volume and Apparent Diffusion Coefficient Quantification. Invest Radiol 52(9):538–546
Chang SS (2004) Overview of prostate-specific membrane antigen. Rev Urol 6(Suppl 10):S13–S18
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C (1997) Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 3(1):81–85
Rauscher I, Maurer T, Beer AJ et al (2016) Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J Nucl Med 57(11):1713–1719
Schwarzenboeck SM, Rauscher I, Bluemel C et al (2017) PSMA Ligands for PET Imaging of Prostate Cancer. J Nucl Med 58(10):1545–1552
Maurer T, Gschwend JE, Rauscher I et al (2016) Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 195(5):1436–1443
Pyka T, Okamoto S, Dahlbender M et al (2016) Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 43(12):2114–2121
Fendler WP, Schmidt DF, Wenter V et al (2016) 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J Nucl Med 57(11):1720–1725
Eiber M, Weirich G, Holzapfel K et al (2016) Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localisation of Primary Prostate Cancer. Eur Urol 70(5):829–836
Hoeks CMA, Barentsz JO, Hambrock T et al (2011) Prostate cancer: multiparametric MR imaging for detection, localisation, and staging. Radiology 261(1):46–66
Turkbey B, Choyke PL (2015) PIRADS 2.0: what is new? Diagn Interv Radiol 21(5):382–384
Kim SH, Choi MS, Kim MJ, Kim YH, Cho SH (2017) Validation of prostate imaging reporting and data system version 2 using an mri-ultrasound fusion biopsy in prostate cancer diagnosis. AJR Am J Roentgenol 209(4):800–805
Borofsky S, George AK, Gaur S, et al. (2017) What are we missing? false-negative cancers at multiparametric mr imaging of the prostate. Radiology 286(1):186–195
Metzger GJ, Kalavagunta C, Spilseth B et al (2016) Detection of Prostate Cancer: Quantitative Multiparametric MR Imaging Models Developed Using Registered Correlative Histopathology. Radiology 279(3):805–816
Isebaert S, Van den Bergh L, Haustermans K et al (2013) Multiparametric MRI for prostate cancer localisation in correlation to whole-mount histopathology. J Magn Reson Imaging 37(6):1392–1401
Pepe P, D’Urso D, Garufi A et al (2017) Multiparametric MRI Apparent Diffusion Coefficient (ADC) Accuracy in Diagnosing Clinically Significant Prostate Cancer. In Vivo 31(3):415–418
Hambrock T, Somford DM, Huisman HJ et al (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
Yoon MY, Park J, Cho JY et al (2017) Predicting biochemical recurrence in patients with high-risk prostate cancer using the apparent diffusion coefficient of magnetic resonance imaging. Investig Clin Urol 58(1):12–19
Kido A, Tamada T, Sone T et al (2017) Incremental value of high b value diffusion-weighted magnetic resonance imaging at 3-T for prediction of extracapsular extension in patients with prostate cancer: preliminary experience. Radiol Med (Torino) 122(3):228–238
Koerber SA, Utzinger MT, Kratochwil C et al (2017) 68Ga-PSMA-11 PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake with Several Clinical Parameters. J Nucl Med (12):1943–1948
Woythal N, Arsenic R, Kempkensteffen C et al (2017) Immunohistochemical validation of PSMA-expression measured by (68)Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med 59(2):238–243
Gibbs P, Liney GP, Pickles MD, Zelhof B, Rodrigues G, Turnbull LW (2009) Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol 44(9):572–576
Uprimny C, Kroiss AS, Decristoforo C et al (2017) Early dynamic imaging in 68Ga- PSMA-11 PET/CT allows discrimination of urinary bladder activity and prostate cancer lesions. Eur J Nucl Med Mol Imaging 44(5):765–775
Domachevsky L, Bernstine H, Goldberg N et al (2017) Early 68GA-PSMA PET/MRI acquisition: assessment of lesion detectability and PET metrics in patients with prostate cancer undergoing same-day late PET/CT. Clin Radiol 72(11):944–950
Acknowledgements
The authors wish to acknowledge Zohar Nitsan, Inbal Machcat and Hagai Baruch for their excellent work in patient scanning, and to Limor Shaharabani-Gargir for patient management.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Domachevsky Liran.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Rights and permissions
About this article
Cite this article
Domachevsky, L., Goldberg, N., Bernstine, H. et al. Quantitative characterisation of clinically significant intra-prostatic cancer by prostate-specific membrane antigen (PSMA) expression and cell density on PSMA-11. Eur Radiol 28, 5275–5283 (2018). https://doi.org/10.1007/s00330-018-5484-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5484-1