Abstract
Objectives
To analyze the utility of metabolic imaging, and specifically of dedicated breast positron emission tomography (dbPET) to differentiate between indolent and potentially aggressive ductal carcinoma in situ (DCIS).
Methods
After institutional review board approval, we retrospectively reviewed the cases of pure DCIS who underwent dbPET before biopsy and surgery in Lucus Augusti Universitary Hospital (Lugo, Spain) and in Fudan Cancer Institute (Shanghai, China) between January 2016 and May 2018. Grade 1 and “non-comedo” grade 2 DCIS were considered low-risk disease, while intermediate-grade with necrosis or grade 3 cases were included in the high-risk group. DbPET sensitivity and specificity to differentiate between indolent and potentially aggressive DCIS were determined along with their respective 95% confidence intervals.
Results
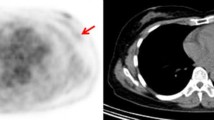
We enrolled 139 surgery-confirmed pure DCIS cases. Fifty were high-risk neoplasms and 89 low-risk DCIS. Only seven low-risk lesions were positive at dbPET and five of potentially aggressive neoplasms did not show FDG uptake, all included into the field of view (FOV). Sensitivity and specificity of dbPET to differentiate between indolent and potentially aggressive DCIS were 90% (95% CI, 77–96%) and 92% (95% CI, 84–97%), respectively.
Conclusion
Metabolic imaging could help to identify the subgroup of indolent lesions from those potentially aggressive ones that may be managed by active surveillance.
Key Points
• Low- and high-grade DCIS likely arise from two distinct evolutionary paths and when low-grade lesions progress to invasive cancer, the tumor is frequently low grade and well differentiated.
• Ongoing clinical trials evaluate whether patients with low-risk DCIS could be safely managed by an active surveillance approach, with avoidance of unnecessary treatments and without impact on ipsilateral invasive breast cancer free survival time.
• Dedicated breast PET may differentiate harmless from potentially hazardous DCIS, supporting active surveillance for the management of those women with low-grade DCIS, decreasing the rate of the upgrade to invasive carcinoma at surgical excision.




Similar content being viewed by others
Abbreviations
- 18F-FDG:
-
18F-fluorodeoxyglucose
- 3D:
-
Three-dimensional
- BC:
-
Breast cancer
- CI:
-
Confidence interval
- dbPET:
-
Dedicated breast PET
- DCE:
-
Dynamic contrast-enhanced
- DCIS:
-
Ductal carcinoma in situ
- ER:
-
Estrogen receptor
- FN:
-
False negative
- FOV:
-
Field of view
- FP:
-
False positive
- FSPGR:
-
Fast spoiled gradient
- GLUT:
-
Glucose transporter
- HER2:
-
Human epidermal growth factor receptor 2
- IBC:
-
Invasive breast cancer
- MAMMI:
-
Mammography with molecular imaging
- MRI:
-
Magnetic resonance imaging
- NCCN:
-
National Comprehensive Cancer Network
- PEM:
-
Positron emission mammography
- PR:
-
Progesterone receptor
- SD:
-
Standard deviation
- SGLT:
-
Sodium-coupled glucose transporter
- STIR:
-
Short-tau inversion recovery
- SUV:
-
Standardized uptake value
- US:
-
Ultrasound
References
van Luijt PA, Heijnsdijk EA, Fracheboud J et al (2016) The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast Cancer Res 18:47–57
Rosner D, Bedwani RN, Vana J, Baker HW, Murphy GP (1980) Noninvasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg 192:139–147
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68:7–30
Blichert-Toft M, Graversen HP, Andersen J, Dyreborg U, Green A (1988) In situ breast carcinomas: a population-based study on frequency, growth pattern, and clinical aspects. World J Surg 12:845–851
Ward BA, McKhann CF, Ravikumar TS (1992) Ten-year follow-up of breast carcinoma in situ in Connecticut. Arch Surg 127:1392–1395
Mardekian SK, Bombonati A, Palazzo JP (2016) Ductal carcinoma in situ of the breast: the importance of morphologic and molecular interactions. Hum Pathol 49:114–123
Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS (2010) Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 57:171–192
Gøtzsche PC, Olsen O (2000) Is screening for breast cancer with mammography justifiable? Lancet 355:129–134
Senkus E, Kyriakides S, Ohno S et al (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl5):v8–v30
Francis A, Thomas J, Fallowfiedl L et al (2015) Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 51:2296–2303
Elshof LE, Tryfonidis K, Slaets L et al (2015) Feasibility of a prospective, randomized, open-label, international multicenter, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ – the LORD study. Eur J Cancer 51:1497–1510
Hwang EF, Hyslop T, Lynch T, et al. (2019) The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 9:e026797
Teixeira SC, Rebolleda JF, Koolen BB et al (2016) Evaluation of a hanging-breast PET system for primary tumor visualization in patients with stage I-III breast cancer: comparison with standard PET/CT. AJR Am J Roentgenol 206:1307–1314
D’Orsi CJ, Sickles EA, Mendelson EB et al (2013) ACR BI-RADS Atlas. Breast imaging reporting and data system. American College of Radiology, Reston
Sanders ME, Schuyler PA, Simpson JF, Page DL, Dupont WD (2015) Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol 28:662–669
Betsill WL Jr, Rosen PP, Lieberman PH, Robbins GF (1978) Intraductal carcinoma. Long-term follow-up after treatment by biopsy alone. JAMA 239:1863–1867
Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ (2005) Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer 103:1778–1784
Reis-Filho JS, Simpson PT, Gale T, Lakhani SR (2005) The molecular genetics of breast cancer: the contribution of comparative genomic hybridization. Pathol Res Pract 201:713–725
Simpson PT, Reis-Filho JS, Gale T, Lakhani SR (2005) Molecular evolution of breast cancer. J Pathol 205:248–254
Kerlikowske K, Molinaro AM, Gauthier ML et al (2010) Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 102:627–637
Sarode VR, Han JS, Morris DH, Peng Y, Rao R (2011) A comparative analysis of biomarker expression and molecular subtypes of pure ductal carcinoma in situ and invasive breast carcinoma by image analysis: relationship of the subtypes with histologic grade, Ki67, p53 overexpression, and DNA ploidy. Int J Breast Cancer 2011:217060
Han K, Nofech-Mozes S, Narod S et al (2012) Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol (R Coll Radiol) 24:183–189
Vincent-Salomon A, Lucchesi C, Gruel N et al (2008) Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res 14:1956–1965
Petridis C, Brook MN, Shah V et al (2016) Genetic predisposition to ductal carcinoma in situ of the breast. Breast Cancer Res 18:22
Carraro DM, Elias EV, Andrade VP (2014) Ductal carcinoma in situ of the breast: morphological and molecular features implicated in progression. Biosci Rep 34:19–28
Patel GV, Van Sant EP, Taback B, Ha R (2018) Patient selection for ductal carcinoma in situ observation trials: are the lesions truly low risk? AJR Am J Roentgenol 211:712–713
Rahbar H, Parsian S, Lam DL et al (2016) Can MRI biomarkers at 3 Tesla identify low risk ductal carcinoma in situ? Clin Imaging 40:125–129
Hussein H, Chung C, Moshonov H, Miller N, Kulkarni SR, Scaranelo AM (2015) Evaluation of apparent diffusion coefficient to predict grade, microinvasion, and invasion in ductal carcinoma in situ of the breast. Acad Radiol 22:1483–1488
Avril N, Rosé CA, Schelling M et al (2006) Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 18:3495–3502
Mavi A, Urhan M, Yu JQ et al (2006) Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 47:1440–1446
Kim S, Lee S, Kim S, Lee S, Yum H (2018) The usefulness of fluorodeoxyglucose-PET/CT for preoperative evaluation of ductal carcinoma in situ. Ann Surg Treat Res 94:63–68
Fujioka T, Kubota K, Toriihara A et al (2016) Tumor characteristics of ductal carcinoma in situ of breast visualizes on [F-18]-fluorodeoxyglucose-positron emission tomography/computed tomography: results from a retrospective study. World J Radiol 8:743–749
Fujii T, Yanai K, Tokuda S et al (2017) Clinicopathological features of ductal carcinoma in situ from 18F-FDG-PET findings. Anticancer Res 37:5053–5056
Fujii T, Yajima R, Tatsuki H, Kuwano H (2017) 18F-fluorodeoxyglucose uptake as predictor for invasion in preoperatively diagnosed breast ductal carcinoma in situ. Significance in cases without mass formation. Mol Clin Oncol 7:183–187
Seo YY, Yoo IR, Park SY, Oh JK, Kim SH, Sohn HS (2016) Ductal carcinoma in situ and ductal carcinoma in situ with microinvasion: correlation of FDG uptake with histological and biological prognostic factors. Breast Cancer 24:353–361
Yoon HJ, Kim Y, Kim BS (2015) Intratumoral metabolic heterogeneity predicts invasive components in breast ductal carcinoma in situ. Eur Radiol 25:3648–3658
Shigematsu H, Kadoya T, Masumoto N et al (2014) Role of FDG-PET/CT in prediction of underestimation of invasive breast cancer in cases of ductal carcinoma in situ diagnosed at needle biopsy. Clin Breast Cancer 14:358–364
Azuma A, Tozaki M, Ito K, Fukuma E, Tanaka T, O’uchi T (2008) Ductal carcinoma in situ: correlation between FDG-PET/CT and histopathology. Radiat Med 26:488–493
Shilling K, Narayanan D, Kalinyak JE et al (2011) Positron emission mammography in breast cancer presurgical planning: comparisons with magnetic resonance imaging. Eur J Nucl Mol Imaging 38:23–36
Bitencourt AG, Lima EN, Macedo BR et al (2017) Can positron emission mammography help to identify clinically significant breast cancer in women with suspicious calcifications on mammography? Eur Radiol 27(5):1893–1900
Soriano A, González A, Otero A et al (2011) Attenuation correction without transmission scan for the MAMMI breast PET. Nucl Inst Methods Phys Res A 648(Suppl1):S75–S78
Koolen BB, Aukema TS, González Martínez AJ et al (2013) First clinical experience with a dedicated PET for hanging breast molecular imaging. Q J Nucl Med Mol Imaging 57:92–100
Graña-López L, Herranz M, Domínguez-Prado I et al (2018) Dedicated breast PET value to evaluate BI-RADS 4 breast lesions. Eur J Radiol 108:201–207
Avril N, Menzel M, Dose J et al (2001) Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med 42:9–16
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL (1996) Intratumoral distribution of tritiated-FDG in breast carcinoma: correlation between Glut-1 expression and FDG uptake. J Nucl Med 37:1042–1047
Jadvar H, Alavi A, Gambhir SS (2009) 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med 50:1820–1827
Yamamoto T, Seino Y, Fukumoto H et al (1990) Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 170:223–230
Taira N, Atsumi E, Nakachi S et al (2018) Comparison of GLUT-1, SGLT-1, and SGLT-2 expression in false–negative and true-positive lymph nodes during the 18F-FDG PET/CT mediastinal nodal staging of non-small cell lung cancer. Lung Cancer 123:30–35
Kuo SJ, Wu YC, Chen CP, Tseng HS, Chen DR (2006) Expression of glucose transporter- 1 in Taiwanese patients with breast carcinoma – a preliminary report. Kaohsiung J Med Sci 22:339–345
Laudanski P, Koda M, Kozłowski L et al (2004) Expression of glucose transporter GLUT-1 and estrogen receptors ER-alpha and ER-beta in human breast cancer. Neoplasma 51:164–168
O’Connor MK, Tran TD, Swanson TN, Ellingson LR, Hunt KN, Whaley DH (2017) Improved visualization of breast tissue on a dedicated breast PET system through ergonomic redesign of the imaging table. EJNMMI Res 7:100
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Manuel Vázquez-Caruncho.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all Spanish subjects (patients) in this study.
For the Chinese women, informed consent was waived because this is a retrospective review of a patient database and no changes on their standard of care were made.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
None study subjects or cohorts have been previously reported.
Methodology
• Retrospective
• Observational
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Graña-López, L., Herranz, M., Domínguez-Prado, I. et al. Can dedicated breast PET help to reduce overdiagnosis and overtreatment by differentiating between indolent and potentially aggressive ductal carcinoma in situ?. Eur Radiol 30, 514–522 (2020). https://doi.org/10.1007/s00330-019-06356-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06356-9